
Part One: The Endocannabinoid System and Autism Spectrum Disorder (ASD)
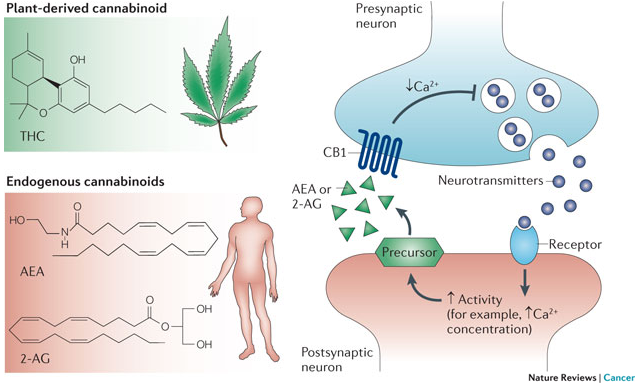
 The importance of the discovery of the role that the endocannabinoid system (ECS) plays in human health and disease cannot be understated. Cannabinoid receptors are the most highly expressed of any G-protein coupled receptor (GPCR) in the body. They’re the only ones to play a direct role in virtually every aspect of the human body (CNS and immune systems, throughout the periphery, presynaptic, and postsynaptic).[1]
The importance of the discovery of the role that the endocannabinoid system (ECS) plays in human health and disease cannot be understated. Cannabinoid receptors are the most highly expressed of any G-protein coupled receptor (GPCR) in the body. They’re the only ones to play a direct role in virtually every aspect of the human body (CNS and immune systems, throughout the periphery, presynaptic, and postsynaptic).[1]
It’s no wonder that anecdotal reports of cannabis treatments indicate effectiveness in such a wide array of conditions. The growing body of scientific research surrounding the endocannabinoid system continues to lead to the further understanding of the physiological basis in a growing number of conditions.[45]
One condition with both supportive anecdotal and preclinical scientific evidence is for patients on the severe end of the autism spectrum (ASD). In a short series of articles we’ll attempt to shed light on the role that the endocannabinoid system plays in the progression of autism, the potential role of phytocannabinoids in treatment, and what that might mean in a practical sense.
NL3 Mutations Inhibit Tonic Endocannabinoid Secretions
Neuroligins are part of a family of neuronal cell surface proteins that “connect presynaptic and postsynaptic neurons at synapses, mediate signaling across the synapse, and shape the properties of neural networks by specifying synaptic functions”. Alterations in genes encoding neuroligins are associated with autism and other cognitive diseases.[56]
Mutations in neuroligin-3 (NL3), a member of the family of neuroligins, are associated with ASD.[17] NL3 is required for tonic secretion of endocannabinoids (AEA, 2-AG).[17] NL3 mutations have been shown to inhibit tonic endocannabinoid secretion.[17] This dysregulation in endocannabinoid signaling may contribute to the pathophysiology of autism.[17, 50, 53] These findings have in part prompted researchers to apply to conduct research with nonhuman primates in order to further elucidate this association.[39]
Targeting Endocannabinoid System to Treat FXS
Fragile X syndrome (FXS) is the most commonly known genetic cause of autism.[30] FXS is associated with a loss of the fragile X mental retardation protein (FMRP) which regulates signal transduction in the brain.[30] This FMRP deficiency is believed to “increase neuronal excitability which is mediated by endocannabinoids”.[59]
FXS is also associated with “neuropsychiatric problems such as hyperactivity, attention disorders, and seizures.”[19] The endocannabinoid system is key to modulating functions that are involved with regulating all of these disorders including “synaptic plasticity, cognitive performance, anxiety, nociception and seizure susceptibility.”[19] The endocannabinoid system is specifically implicated in just about all aspects of FXS including “behavioral, synaptic and molecular manifestations.”[19] Preclinical research implicates CB1 and CB2 as pharmacological targets with the potential to reduce cognitive deficits and anxiety in FXS models in rodents.[19, 59]
Increased Expression of CB2 Receptors Associated with ASD
Though it wasn’t long ago that the role that CB2 receptors played in the human brain was believed to be negligible, additional research has implicated it as having a much more substantial role than previously understood.
“Given that CB2 is up-regulated, and that it’s believed to play a neuroprotective role, CB2 is being investigated as a potential target for treatment of ASD.[53]”
One example is that CB2 is believed to play a neuroprotective role in response to a variety of inflammatory stimuli, this has implications in a number of neuropsychiatric conditions including ASD.[4, 16, 53]
In ASD, as well as a number of conditions, the expression level of CB2 receptors increases in response to the inflammatory nature of the condition.[16, 53] Given that CB2 is up-regulated, and that it’s believed to play a neuroprotective role, CB2 is being investigated as a potential target for treatment of ASD.[53]
Elevated Cytokine Levels Associated with ASD
“Cytokines are small secreted proteins released by cells that have a specific effect on the interactions and communications between cells… Pro-inflammatory cytokines are involved in the up-regulation of inflammatory reactions.”[60]
Elevated pro-inflammatory cytokine levels are associated with ASD.[44] Whether this is due in part as a result of NL3 mutations inhibiting tonic secretion of endocannabinoids remains uncertain. However, endocannabinoids (AEA, 2-AG) have been shown to play key roles inhibiting cytokines via CB2.[12, 47]
The majority of cannabinoids have been demonstrated to decrease cytokine production via CB1/CB2 dependent and independent mechanisms.[25, 27, 29, 36]
Clinically Diagnosing ASD via the ECS
A team of researchers recently discovered and patented a process that claims that it’s possible to clinically diagnose ASD, and susceptibility to it, via observation of the degree of modulation that acetaminophen has on endocannabinoid levels. However, based on a series of deductions made within their published literature, it appears that additional research is required.
Other Relevant ECS/ASD Implications
The number of functions that ECS regulate is extensive and beyond the scope of this paper.[45, 48] However, a few potentially relevant aspects to ASD will be listed:
- “CB1 variations modulate the striatal function that underlies the perception of signals of social reward, such as happy faces. This suggests that CB1 is a key element in the molecular architecture of perception of certain basic emotions. This may have implications for understanding neurodevelopmental conditions marked by atypical eye contact and facial emotion processing, such as ASC.”[13]
- “Endocannabinoids are key modulators of synaptic function.”[11]
- Tonic secretions of endocannabinoids regulate GI functions (including metabolism).[15, 37]
- Endocannabinoids regulate stress responses, in part via the modulation of the 5-HT system.[23]
- Additional targets of endocannabinoids (and exogenous cannabinoids), PPARα, PPARγ, and GPR55 expression levels have shown reductions in a valproic acid model of autism in rats.[33]
Conclusion
Based on the preclinical research the endocannabinoid system appears to be directly impacted by, as well as a potential target for treatment of, physiological manifestations of genetic factors associated with ASD including NL3 mutations and FXS. NL3 mutations inhibit tonic secretion of endocannabinoids and disrupt their signaling. This possibly contributes to the identified increase in pro-inflammatory cytokines levels in ASD. CB2 is upregulated in the brain in response to inflammatory stimuli as part of a neuroprotective role, and is suggested as a target for treatment. There appears to be a preponderance of evidence that the ECS is involved in the progression of ASD.
Explore the similarities between phytocannabinoids and endocannabinoids, as well as any potential practical applications cannabinoids might provide for treatment of ASD in the next article in this series.
Citations & References
There are 60 references in this article. Click here to view them all.
- Alger, Bradley. "Getting High on the Endocannabinoid System." Cerebrum (2013).
- Andó, Rómeó D., et al. "The inhibitory action of exo-and endocannabinoids on [3H] GABA release are mediated by both CB1 and CB2 receptors in the mouse hippocampus." Neurochemistry International 60.2 (2012): 145-152.
- Aso, Ester, et al. "Lack of CB1 receptor activity impairs serotonergic negative feedback." Journal of neurochemistry 109.3 (2009): 935-944.
- Benito, C., et al. "Cannabinoid CB2 receptors in human brain inflammation." British journal of pharmacology 153.2 (2008): 277-285.
- Best, Aaron R., and Wade G. Regehr. "Serotonin evokes endocannabinoid release and retrogradely suppresses excitatory synapses." The Journal of Neuroscience 28.25 (2008): 6508-6515.
- Bolognini, D., et al. "Cannabidiolic acid prevents vomiting in Suncus murinus and nausea‐induced behaviour in rats by enhancing 5‐HT1A receptor activation." British journal of pharmacology 168.6 (2013): 1456-1470.
- Booz, George W. "Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress." Free Radical Biology and Medicine 51.5 (2011): 1054-1061.
- Braida, Daniela, et al. "5-HT1A receptors are involved in the anxiolytic effect of ∆9-tetrahydrocannabinol and AM 404, the anandamide transport inhibitor, in Sprague–Dawley rats." European journal of pharmacology 555.2 (2007): 156-163.
- Carey, Megan R., et al. "Presynaptic CB1 receptors regulate synaptic plasticity at cerebellar parallel fiber synapses." Journal of neurophysiology 105.2 (2011): 958.
- Carley, David W., et al. "Functional role for cannabinoids in respiratory stability during sleep." Sleep 25.4 (2002): 391-398.
- Castillo, Pablo E., et al. "Endocannabinoid signaling and synaptic function." Neuron 76.1 (2012): 70-81.
- Cencioni, Maria Teresa, et al. "Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors." PLoS One 5.1 (2010): e8688.
- Chakrabarti, Bhismadev, and Simon Baron-Cohen. "Variation in the human Cannabinoid Receptor (CNR1) gene modulates gaze duration for happy faces." Molecular autism 2.1 (2011): 10.
- Di Filippo, Clara, et al. "Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: involvement of cytokine/chemokines and PMN." Journal of leukocyte biology 75.3 (2004): 453-459.
- Di Marzo, V., and F. Piscitelli. "Gut feelings about the endocannabinoid system." Neurogastroenterology & Motility 23.5 (2011): 391-398.
- Fernández‐Ruiz, Javier, et al. "Prospects for cannabinoid therapies in basal ganglia disorders." British journal of pharmacology 163.7 (2011): 1365-1378.
- Földy, Csaba, Robert C. Malenka, and Thomas C. Südhof. "Autism-associated neuroligin-3 mutations commonly disrupt tonic endocannabinoid signaling." Neuron 78.3 (2013): 498-509.
- Gamage, Thomas F., and Aron H. Lichtman. "The endocannabinoid system: role in energy regulation." Pediatric blood & cancer 58.1 (2012): 144-148.
- Garcia, Arnau Busquets, et al. “New insights into the molecular pathophysiology of fragile X syndrome and therapeutic perspectives from the animal model”, International Journal of Biochemistry and Cell Biology, 53 (2014) 121-126.
- Godlewski, Grzegorz, Manfred Göthert, and Barbara Malinowska. "Cannabinoid receptor‐independent inhibition by cannabinoid agonists of the peripheral 5‐HT3 receptor‐mediated von Bezold–Jarisch reflex." British journal of pharmacology 138.5 (2003): 767-774.
- Gomes, Felipe V., Leonardo BM Resstel, and Francisco S. Guimarães. "The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors." Psychopharmacology 213.2-3 (2011): 465-473.
- Gong, Jian-Ping, et al. "Cannabinoid CB2 receptors: immunohistochemical localization in rat brain." Brain research 1071.1 (2006): 10-23.
- Haj-Dahmane, Samir, and Roh-Yu Shen. "Modulation of the serotonin system by endocannabinoid signaling." Neuropharmacology 61.3 (2011): 414-420.
- Iring, András, et al. "Role of Endocannabinoids and Cannabinoid-1 Receptors in Cerebrocortical Blood Flow Regulation." PloS one 8.1 (2013): e53390.
- Izzo, Angelo A., et al. "Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb." Trends in pharmacological sciences 30.10 (2009): 515-527.
- Jean-Gilles, Lucie, Bruno Gran, and Cris S. Constantinescu. "Interaction between cytokines, cannabinoids and the nervous system." Immunobiology 215.8 (2010): 606-610.
- Jiang, Chengyu, Adrian T. Ting, and Brian Seed. "PPAR-γ agonists inhibit production of monocyte inflammatory cytokines." Nature 391.6662 (1998): 8286.
- Johnson, Jeremy R., et al. "Multicenter, double-blind, randomized, placebocontrolled, parallel-group study of the efficacy, safety, and tolerability of THC: CBD extract and THC extract in patients with intractable cancer-related pain." Journal of pain and symptom management 39.2 (2010): 167-179.
- Juknat, Ana, et al. "Cannabidiol affects the expression of genes involved in zinc homeostasis in BV-2 microglial cells." Neurochemistry international 61.6 (2012): 923-930.
- Jung, Kwang-Mook, et al. "Uncoupling of the endocannabinoid signalling complex in a mouse model of fragile X syndrome." Nature communications 3 (2012): 1080.
- Katona, István, and Tamás F. Freund. "Multiple functions of endocannabinoid signaling in the brain." Annual review of neuroscience 35 (2012): 529-558.
- Kawamura, Yoshinobu, et al. "The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum." The Journal of neuroscience 26.11 (2006): 2991-3001.
- Kerr, D. M., et al. "Alterations in the endocannabinoid system in the rat valproic acid model of autism." Behavioural brain research 249 (2013): 124-132.
- Kishimoto, Yasushi, and Masanobu Kano. "Endogenous cannabinoid signaling through the CB1 receptor is essential for cerebellum-dependent discrete motor learning." The Journal of neuroscience 26.34 (2006): 8829-8837.
- Klegeris, Andis, Christopher J. Bissonnette, and Patrick L. McGeer. "Reduction of human monocytic cell neurotoxicity and cytokine secretion by ligands of the cannabinoid‐type CB2 receptor." British journal of pharmacology 139.4 (2003): 775-786.
- Kozela, Ewa, et al. "Cannabinoids ∆9-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-κB and interferonβ/STAT proinflammatory pathways in BV-2 microglial cells." Journal of biological chemistry 285.3 (2010): 1616-1626.
- Li, Chen, Peter M. Jones, and Shanta J. Persaud. "Role of the endocannabinoid system in food intake, energy homeostasis and regulation of the endocrine pancreas." Pharmacology & therapeutics 129.3 (2011): 307-320.
- Machado Bergamaschi, Mateus, et al. "Safety and side effects of cannabidiol, a Cannabis sativa constituent." Current drug safety 6.4 (2011): 237-249.
- Malcher-Lopes, Renato. "Targeting alterations in the endocannabinoid system of rodents and non-human primates for the study of autism." Qatar Foundation Annual Research Conference. No. 2013. 2013.
- Marco, Eva M., et al. "Endocannabinoid system and psychiatry: in search of a neurobiological basis for detrimental and potential therapeutic effects." Frontiers in behavioral neuroscience 5 (2011).
- Mato, Susana, et al. "CB1 knockout mice display impaired functionality of 5‐HT1A and 5‐HT2A/C receptors." Journal of neurochemistry 103.5 (2007): 2111-2120.
- Mikics, Eva, et al. "Interactions between the anxiogenic effects of CB1 gene disruption and 5-HT3 neurotransmission." Behavioural pharmacology 20.3 (2009): 265-272.
- Müller‐Vahl, K. R., et al. "Cannabinoids: possible role in patho‐physiology and therapy of Gilles de la Tourette syndrome." Acta Psychiatrica Scandinavica 98.6 (1998): 502-506.
- Napolioni, Valerio, et al. "Plasma cytokine profiling in sibling pairs discordant for autism spectrum disorder." Journal of neuroinflammation 10.1 (2013): 38.
- Pacher, Pál, Sándor Bátkai, and George Kunos. "The endocannabinoid system as an emerging target of pharmacotherapy." Pharmacological reviews 58.3 (2006): 389-462.
- Palazuelos, Javier, et al. "CB2 cannabinoid receptors promote neural progenitor cell proliferation via mTORC1 signaling." Journal of Biological Chemistry 287.2 (2012): 1198-1209.
- Panikashvili, David, et al. "The endocannabinoid 2-AG protects the blood–brain barrier after closed head injury and inhibits mRNA expression of proinflammatory cytokines." Neurobiology of disease 22.2 (2006): 257-264.
- Pertwee, R. G., et al. "International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2." Pharmacological reviews 62.4 (2010): 588-631.
- Pomerantz, Daniel J. "THE ROLE OF CB2 ENDOCANNABINOID RECEPTOR AND MTORC1 IN NEUROPROGENITOR CELL PROLIFERATION IN TUBEROUS SCLEROSIS." Emphasis Program (2013): 73.
- Onaivi, E. S., et al. "Consequences of cannabinoid and monoaminergic system disruption in a mouse model of autism spectrum disorders." Current neuropharmacology 9.1 (2011): 209.
- Rock, Erin. Cannabidiol Indirectly Activates 5-HT1A Somatodendritic Autoreceptors to Attenuate Vomiting and Nausea. Diss. 2011.
- Roloff, Alan M., et al. "Homer 1a gates the induction mechanism for endocannabinoid-mediated synaptic plasticity." The Journal of Neuroscience 30.8 (2010): 3072-3081.
- Siniscalco, Dario, et al. "Cannabinoid receptor type 2, but not type 1, is upregulated in peripheral blood mononuclear cells of children affected by autistic disorders." Journal of autism and developmental disorders 43.11 (2013): 26862695.
- Sharkey, Keith A., Nissar A. Darmani, and Linda A. Parker. "Regulation of nausea and vomiting by cannabinoids and the endocannabinoid system." European journal of pharmacology 722 (2014): 134-146.
- Stone, Joe, et al. “Cannabinoids, Ketogenic Diets, Holy Basil, and the PPAR Connection.” Unpublished (2014) http://www.scribd.com/doc/207827158/Cannabinoids-Ketogenic-Diets-HolyBasil-and-the-PPAR-Connection
- Sudhof, Thomas C., et al. “Neuroligins and neurexins link synaptic function to cognitive disease”, Nature, 2008/10/16/print, Nature Publishing Group, http://dx.doi.org/10.1038/nature07456
- Tanimura, Asami, et al. "Not glutamate but endocannabinoids mediate retrograde suppression of cerebellar parallel fiber to Purkinje cell synaptic transmission in young adult rodents." Neuropharmacology 57.2 (2009): 157-163.
- Vaney, C., et al. "Efficacy, safety and tolerability of an orally administered cannabis extract in the treatment of spasticity in patients with multiple sclerosis: a randomized, double-blind, placebo-controlled, crossover study." Multiple Sclerosis 10.4 (2004): 417-424.
- Zhang Longhua, Alger Bradley E., et al. “Enhanced Endocannabinoid Signaling Elevates Neuronal Excitability in Fragile X Syndrome” 2010
- Zhang, Jun-Ming, and Jianxiong An., et al. “Cytokines, Inflammation and Pain.” International anesthesiology clinics2 (2007): 27–37. PMC. Web. 11 June 2015.
Christian Bogner, M.D is fighting to show the world the benefits of Medical Cannabis for the Autism Spectrum. He is also the author of “The Endocannabinoid System as it Relates to Autism” and Medical Jane …
